Researchers within the Mancini Group use the molecular control of chemistry to effect macroscopic changes in biological systems. In the lab, students leverage expertise in organic chemistry, polymer chemistry, and chemical biology to advance several key research areas of synthetic immunology. Currently, we are creating new molecules, both big and small, that direct the immune system through novel mechanisms of action. Using the knowledge gained from these studies we are developing new therapies for maladies ranging from cancer to asthma. The following are a few examples of our approaches to generating novel immune modulating materials:
Research Areas
Bystander Assisted ImmunoTherapy
Our lab has developed an approach to cancer therapy that exploits metabolism and trafficking mechanisms unique to multidrug resistant cancers to elicit an anti-cancer immune response. Cancers are highly heterogeneous in nature which in part accounts for their difficulty in treatment. While many cancers are initially susceptible to chemotherapy, over time, chronic exposure to treatment, provides epigenetic pressure for survival of cancer cell populations that are resistant to chemotherapy. This results in recurrence of multidrug resistant cancers that no longer respond to traditional chemotherapies. Two changes to cancer cell metabolism and molecular trafficking are common among multidrug resistant cancers that allow them to resist traditional chemotherapy: 1) chemotherapeutics are degraded through upregulated hydrolase enzymes such as galactosidase or mannosidase and 2) chemotherapeutic drugs are trafficked outside of cancer cells by proteins involved in active transport, such a P-Glycoproteins. Both of these pathways act in tandem to diminish the anti-cancer effects of chemotherapeutic drugs.
Our Bystander Assisted ImmunoTherapy (BAIT), technique exploits these changes in cancer cell metabolism and molecular trafficking to elicit an anti-cancer immune response. We hypothesized that the altered enzymatic activity of multidrug resistant cancers could be used to convert an inactive prodrug to a potent immunostimulant that could raise an anti-cancer immune response following trafficking to the extracellular space. If successful, this approach would not only produce anti-cancer effects, but would also provide epigenetic pressure to reverse the multidrug resistant phenotype thereby restoring sensitivity to more established chemotherapeutics. To achieve this, the BAIT prodrug needs to both be converted to active immunostimulant by hydrolase enzymes, and undergo drug efflux by P-Glycoproteins, both of which are up-regulated in multidrug resistant cancers. To satisfy the requirements for hydrolase conversion of prodrug to immunostimulant, we are developing several examples of hydrolase labile substrates that can act as a molecular switch to modulate the activity of immunostimulants. This results in enzyme-directed immunostimulants that activate nearby (bystander) immune cells exclusively in the presence of cancer cells that possess complimentary enzyme. To allow this to occur, we are simultaneously developing a library of structural parameters that establish a structure activity relationship for the susceptibility of immunostimulants to P-glycoprotein mediated trafficking. Building on these preliminary studies, synthetic and computational efforts are underway to rationally design new immunostimulant prodrugs that are amenable to the tandem enzymatic conversion and P-glycoprotein trafficking germane to the BAIT technique.
Polymeric Immunomodulators
Covalently linking multiple immune modulating proteins together dramatically alters the immunogenicity of the resulting conjugate. For example, when the immune modulating proteins of Granulocyte Macrophage Colony Stimulating Factor (GMCSF) and various InterLeukins (ILs) are combined, the resulting GMCSF-IL Fusion Transgene (GIFT) conjugates are immunogenic to such a magnitude so as to overcome the immunosupressive effects of cancers and parasitic pathogens alike. Unsurprisingly, this same spatial confinement is also found in the immune modulating proteins expressed on the cell surface of antigen presenting innate immune cells. However, because the synthetic production of heterodimeric protein-polymer conjugates requires multiple synthesis and purification steps, these types of conjugates are currently only produced through bioengineered expression systems that fundamentally limit the possibility for production and structural exploration of the immunogenic heterodimer chemical space.
To circumvent this problem, we have developed a new synthetic polymerization methodology to produce polymer linked α,ω-heterodimer conjugates in one-pot. To do this, we exploit known differences in kinetic parameters between a variety of common monomers and initiators in Atom Transfer Radical Polymerization to effect asymmetric radical-radical recombination of a protein-polymer conjugate and a second protein of interest. We begin by grafting from an immunogenic protein of interest followed by selective termination of growing polymer chains via asymmetric coupling with a second protein to complete the protein-polymer heterodimer conjugate. Using this newly developed polymer chemistry methodology in tandem grafting-from and grafting-to reactions allows us to generate synthetic protein-polymer heterodimers in one-pot reaction sequences. Because our technique is fully synthetic, we can generate immune modulating materials with structural motifs not currently achievable in bioengineered systems. This has resulted in synthetic GIFT conjugates with improved immunogenicity as well as protein-polymer scaffolds that mimic the immune synapse; a first step towards the creation of synthetic antigen presenting cells.
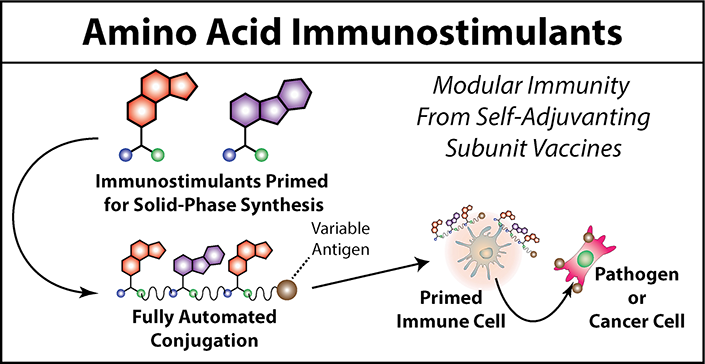
Amino Acid Immunostimulants
Many structural parameters such as multivalency, molecular weight, and inter-agonist density, have been explored extensively in the rational design of macromolecular immunostimulants. Although general trends have been observed for each of these parameters, most observations are empirical; libraries of a small number of compounds (<10) are typically tested for immunogenic properties while attempting to rationalize any observable trends. Because specialty chemistry or bioconjugation techniques are used to produce each of these molecules, library sizes and by extension the observable trends, are limited. Therefore, using a technique that is amenable to automation and multiplexing would help further our understanding of how each of these parameters contribute to immunogenicity. Furthermore, structural trends observed from this study could be optimized for immunogenicity to generate an anti-cancer vaccine that overcomes the immunosuppressive effects of established cancers.
Solid-phase peptide synthesis is one such technique that is amenable to automation and multiplexing. As such we hypothesized that making immunostimulants amenable to solid phase peptide synthesis would enable more extensive exploration of the immunostimulant chemical space ultimately providing better understanding for how each structural parameter contributes to immunogenicity. To do this we conjugate immunostimulants to the side-chain of amino acids. This results in amino acid immunostimulants, effectively a chemical reagent that is primed for solid-phase peptide synthesis. These amino acid immunostimulants can be incorporated into various immunostimulant sequences the same way amino acids are built into peptides by solid-phase peptides synthesis. Furthermore, amino acid immunostimulants can be conjugated directly to peptide antigens of interest resulting in a more potent immune response. Our findings in this area will contribute to the rational design of multivalent immunostimulants used in the next generation of self-adjuvanting vaccines.